RYAN – Temperature impacts the environmental suitability for malaria transmission by Anopheles Gambian and Anopheles stephensi
Oswaldo C. Villena, Sadie J. Ryan, Courtney C. Murdock, Leah R. Johnson
Article first published online: 22 March 2022
DOI: https://doi.org/10.1002/ecy.3685
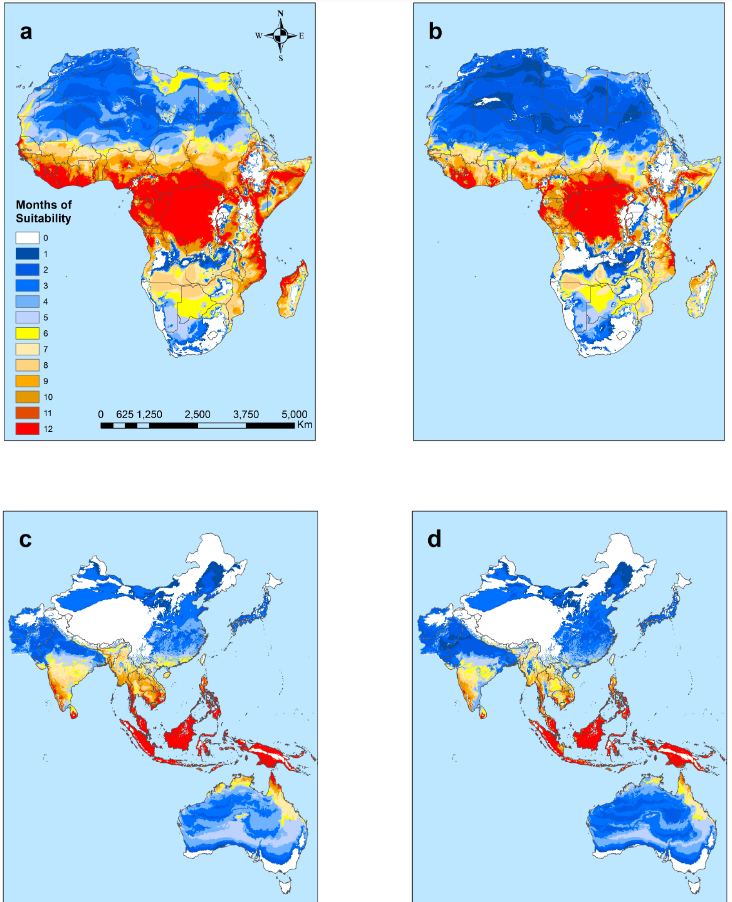
ABSTRACT: Extrinsic environmental factors influence the spatio-temporal dynamics of many organisms, including insects that transmit the pathogens responsible for vector-borne diseases (VBDs). Temperature is an especially important constraint on the fitness of a wide variety of ectothermic insects. A mechanistic understanding of how temperature impacts traits of ectotherms and thus the distribution of ectotherms and vector-borne infections is key to predicting the consequences of climate change on transmission of VBDs like malaria. However, the response of transmission to temperature and other drivers is complex, as thermal traits of ectotherms are typically non-linear, and they interact to determine transmission constraints. In this study, we assess and compare the effect of temperature on the transmission of two malaria parasites, Plasmodium falciparum and Plasmodium vivax, by two malaria vector species, Anopheles gambiae and Anopheles stephensi. We model the non-linear responses of temperature dependent mosquito and parasite traits (mosquito development rate, bite rate, fecundity, proportion of eggs surviving to adulthood, vector competence, mortality rate, and parasite development rate) and incorporate these traits into a suitability metric based on a model for the basic reproductive number across temperatures. Our model predicts that the optimum temperature for transmission suitability is similar for the four mosquito-parasite combinations assessed in this study, but may differ at the thermal limits. More specifically, we found significant differences in the upper thermal limit between parasites spread by the same mosquito (An. stephensi) and between mosquitoes carrying P. falciparum. In contrast, at the lower thermal limit the significant differences were primarily between the mosquito species that both carried the same pathogen (e.g., An. stephensi and An. gambiae both with P. falciparum). Using prevalence data, we show that the transmission suitability metric S(T) calculated from our mechanistic model is consistent with observed P. falciparum prevalence in Africa and Asia but is equivocal for P. vivax prevalence in Asia, and inconsistent with P. vivax prevalence in Africa. We mapped risk to illustrate the number of months various areas in Africa and Asia predicted to be suitable for malaria transmission based on this suitability metric. This mapping provides spatially explicit predictions for suitability and transmission risk.
Read the full publication in Ecology.