BLUHM, NORRIS – Identification of antiviral antihistamines for COVID-19 repurposing
Leah R.Reznikova, Michael H.Norris, Rohit Vashisht, Andrew P. Bluhm, Danmeng Li, Yan-Shin J. Liaoa, Ashley Browne, Atul J.Butte, and David A.Ostrov
Article first published online: 3 DEC 2020 Biochemical and Biophysical Research Communications
DOI: 10.1016/j.bbrc.2020.11.095
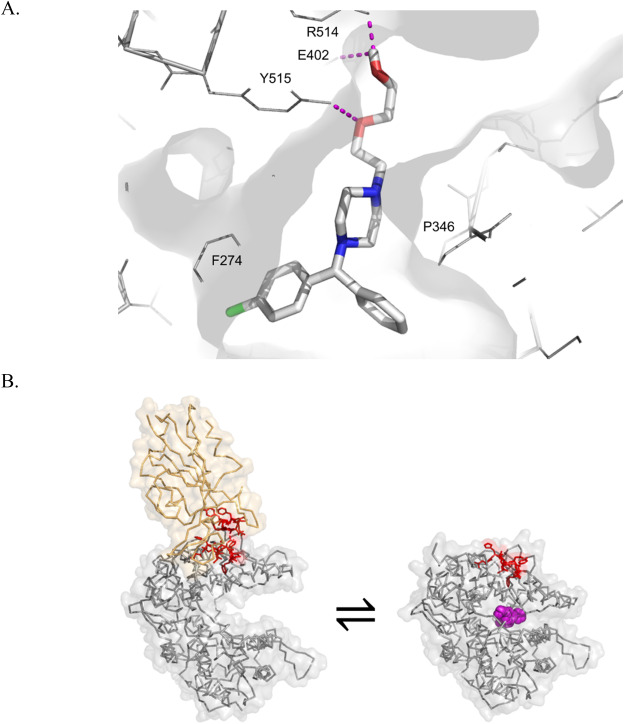
ABSTRACT: There is an urgent need to identify therapies that prevent SARS-CoV-2 infection and improve the outcome of COVID-19 patients. Although repurposed drugs with favorable safety profiles could have significant benefit, widely available prevention or treatment options for COVID-19 have yet to be identified. Efforts to identify approved drugs with in vitro activity against SARS-CoV-2 resulted in identification of antiviral sigma-1 receptor ligands, including antihistamines in the histamine-1 receptor binding class. We identified antihistamine candidates for repurposing by mining electronic health records of usage in population of more than 219,000 subjects tested for SARS-CoV-2. Usage of diphenhydramine, hydroxyzine and azelastine was associated with reduced incidence of SARS-CoV-2 positivity in subjects greater than age 61. We found diphenhydramine, hydroxyzine and azelastine to exhibit direct antiviral activity against SARS-CoV-2 in vitro. Although mechanisms by which specific antihistamines exert antiviral effects is not clear, hydroxyzine, and possibly azelastine, bind Angiotensin Converting Enzyme-2 (ACE2) and the sigma-1 receptor as off-targets. Clinical studies are needed to measure the effectiveness of diphenhydramine, hydroxyzine and azelastine for disease prevention, for early intervention, or as adjuvant therapy for severe COVID-19.
Read the full publication at Biochemical and Biophysical Research Communications