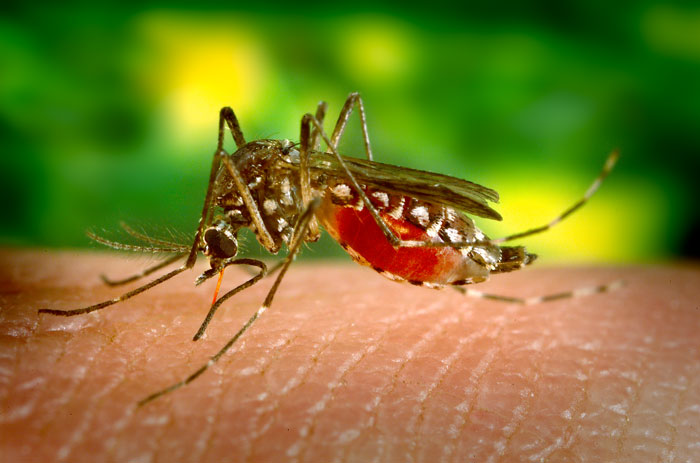
New research models temperature-driven Zika, dengue, and chikungunya transmission
GAINESVILLE, FL – New research co-authored by UF Geography’s Dr. Sadie Ryan and Ms. Cat Lippi sheds light on the climate suitability for Aedes aegypti and Aedes albopictus mosquitos and transmission rates of Zika, chikungunya, and dengue fever. The study, published in PLOS Neglected Tropical Diseases compares new data driven models of Zika, chikungunya, and dengue […]
Read more "New research models temperature-driven Zika, dengue, and chikungunya transmission"