Flying Under the Radar
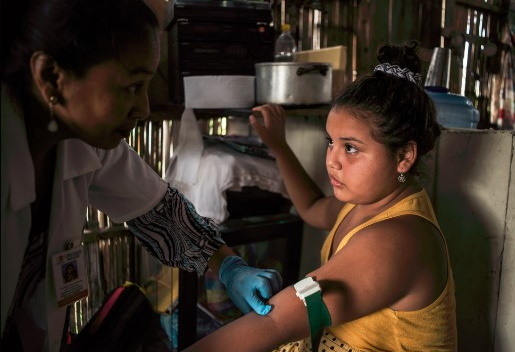
GAINESVILLE, FL – University of Florida Associate Professor of Medical Geography Dr. Sadie Ryan (Emerging Pathogens Institute, Quantitative Disease Ecology & Conservation Lab) spoke with WUFT News about vector-borne disease in the time of coronavirus in Flying Under the Radar. “It’s not like mosquitoes went on hold because of COVID,” she said. “The message needs to stay alive.” […]